3 SEPARATION SYSTEMS
Featured Products
Highest quality standards are achieved through the implementations of latest technology, decades of experience and everlasting moral values , which have helped us to retain our customers as well as multiply them.
Welcome to 3 SEPARATION SYSTEMS
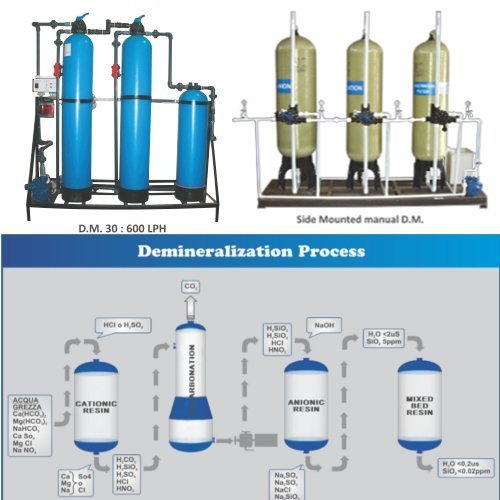
Water Softener

Every household and every factory uses water, and none of it is pure. One class of impurity that is of special interest is "hardness". This refers to the presence of dissolved ions, mainly of calcium Ca2+ and magnesium Mg2+which are acquired through contact with rocks and sediments in the environment. The positive electrical charges of these ions are balanced by the presence of anions (negative ions), of which bicarbonate HCO3– and carbonate CO32– are most important. These ions have their origins in limestone sediments and also from carbon dioxide which is present in all waters exposed to the atmosphere and especially in groundwater.
These "hardness ions" cause two major kinds of problems. First, the metal cations react with soaps, causing them to form an unsightly precipitate— the familiar "bathtub ring". More seriously, the calcium and magnesium carbonates tend to precipitate out as adherent solids on the surfaces of pipes and especially on the hot heat exchanger surfaces of boilers. The resulting scale buildup can impede water flow in pipes. In boilers, the deposits act as thermal insulation that impedes the flow of heat into the water; this not only reduces heating efficiency, but allows the metal to overheat, which in pressurized systems can lead to catastrophic failure.
Types of water hardness:
Temporary hardness:
This refers to hardness whose effects can be removed by boiling the water in an open container. Such waters have usually percolated though limestone formations and contain bicarbonate HCO3– along with small amounts of carbonate CO32– as the principal negative ions. Boiling the water promotes the reaction
2 HCO3– → CO32– + CO2
by driving off the carbon dioxide gas. The CO32– reacts with Ca2+ or Mg2+ ions, to form insoluble calcium and magnesium carbonates which precipitate out. By tying up the metal ions in this way, the amounts available to form soap scum are greatly reduced.
Permanent Hardness:
Waters that contain calcium and magnesium cannot be re mediated by boiling, and are said to be "permanently" hard. The only practical treatment is to remove all the ions, normally by the method described below.
Conventional Water Softening:
Most conventional water-softening devices depend on a process known as ion-exchange in which "hardness" ions trade places with sodium and chloride ions that are loosely bound to an ion-exchange resin or a zeolite(many zeo lite minerals occur in nature, but specialized ones are often made artificially.)
The illustration depicts a negatively-charged zeolite to which [positive] sodium ions are attached. Calcium or magnesium ions in the water displace sodium ions, which are released into the water. In a similar way, positively-charged zeolites bind negatively-charged chloride ions (Cl–), which get displaced by bicarbonate ions in the water. As the zeolites become converted to their Ca2+ and HCO3– forms they gradually lose their effectiveness and must be regenerated. This is accomplished by passing a concentrated brine solution though them, causing the above reaction to be reversed. Herein lies one of the drawbacks of this process: most of the salt employed in the regeneration process gets flushed out of the system and and is usually released into the soil or drainage system— something that can have damaging consequences to the environment, especially in arid regions. For this reason, many jurisdictions prohibit such release, and require users to dispose of the spent brine at an approved site or to use a commercial service company.
Our Vision
* To embrace new technologies and methods. * To give unsurpassed products and services to the clients. * To constantly look for improvement and changes.